
At Vendozi, we provide advanced technologies to support processes related to laboratory and imaging diagnostics in clinical trials.
Our partners










How do we operate?
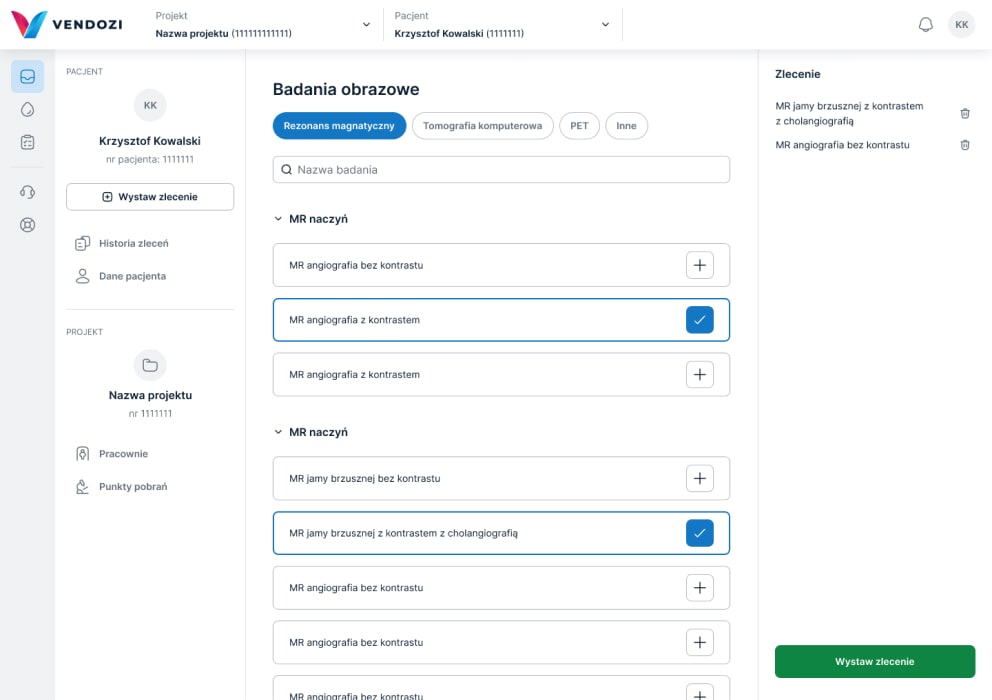
Imaging diagnostics
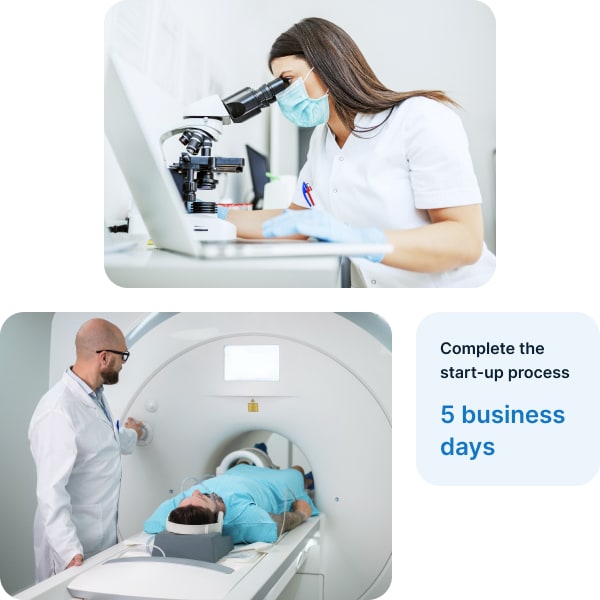
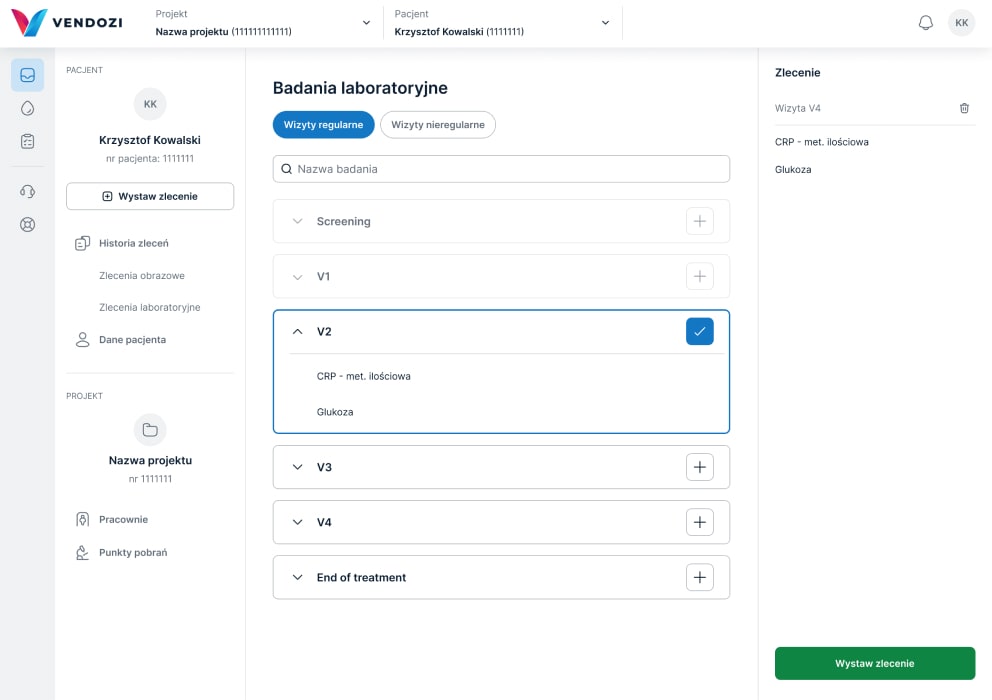
Laboratory diagnostics

Start-up
Wide range of laboratory and radiological procedures
Comprehensive list of over 1300 specimen collection points and more than 1000 diagnostic imaging facilities
Comprehensive technical documentation and validation of medical staff credentials
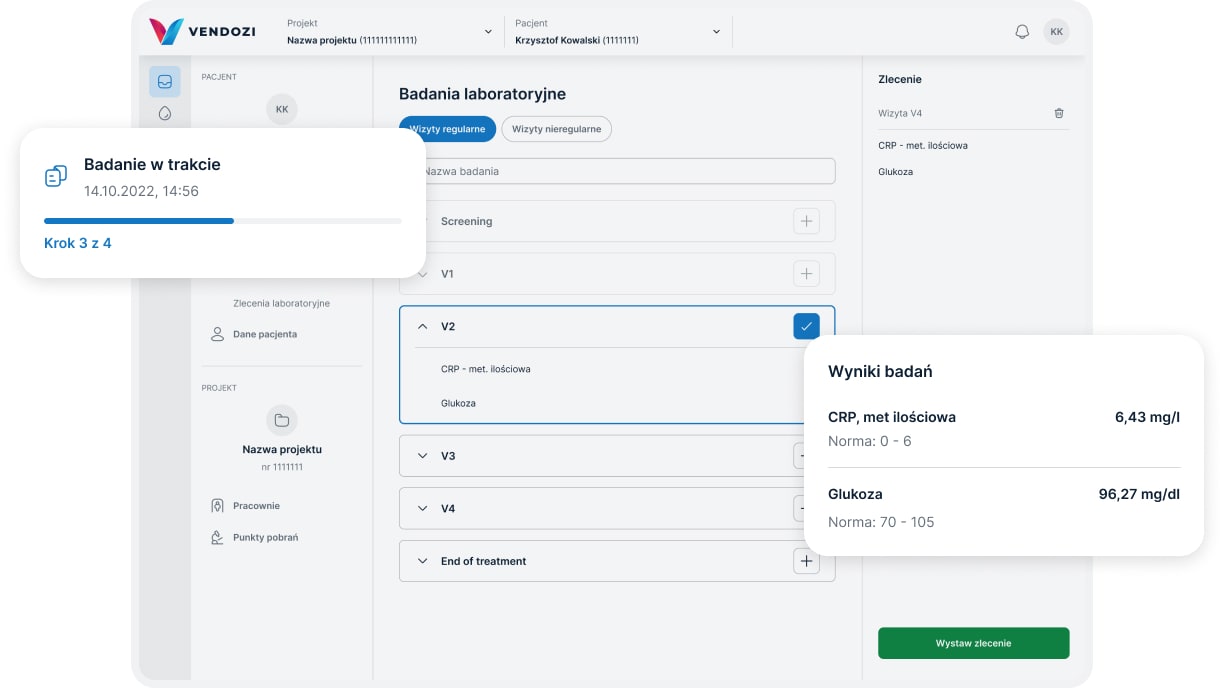
Commissioning examinations
The Vendozi application enables the commissioning of local laboratory and imaging examinations in clinical trials.
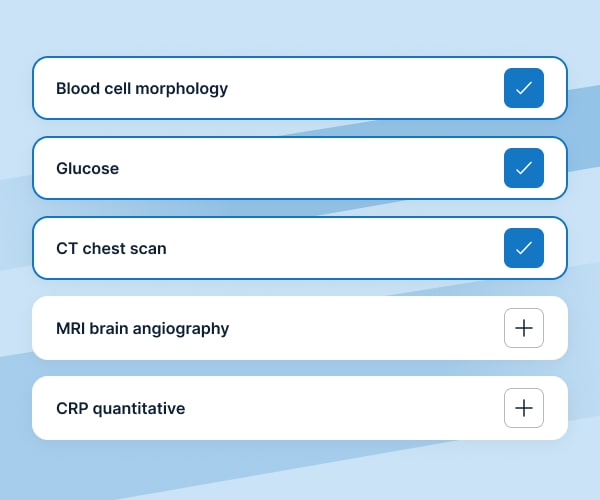
We provide over 1500 specialized laboratory tests as well as a full range of CT/MR/PET-CT scans.
Researchers and coordinators have the option to create their own examination packages.
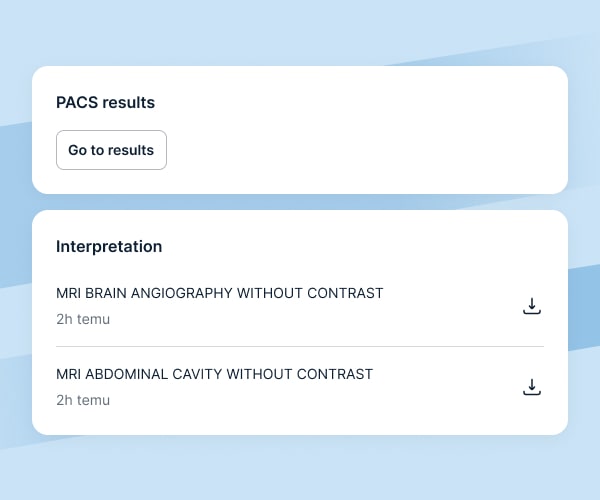
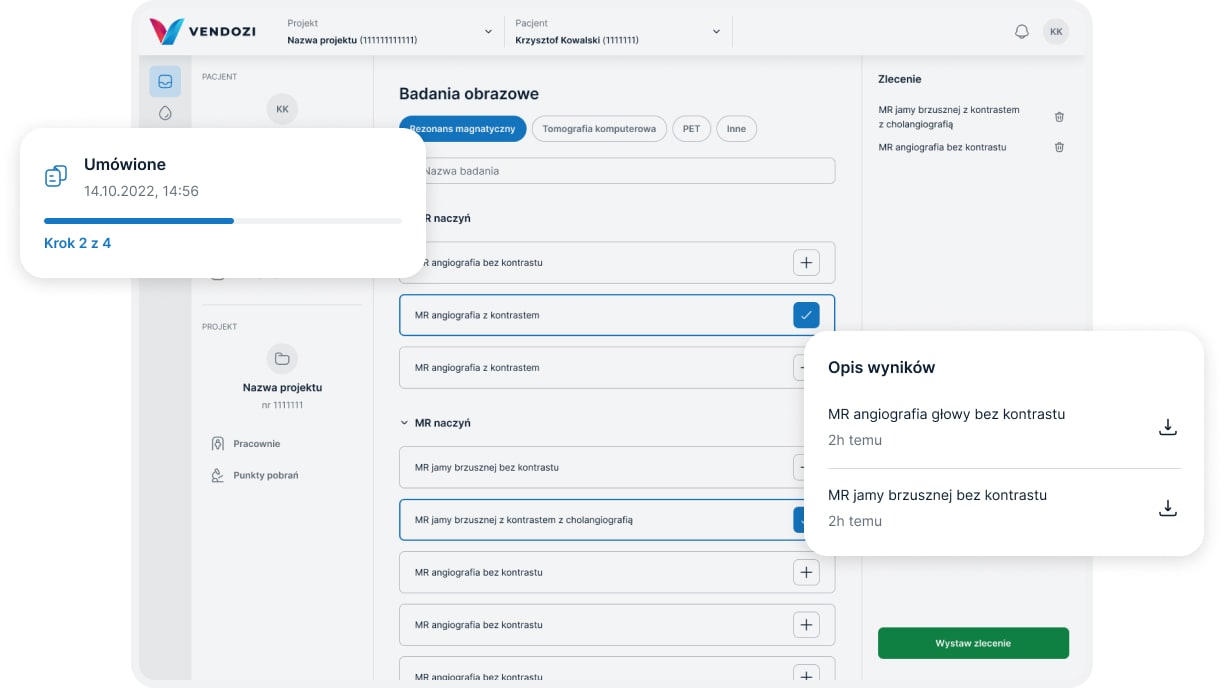
Examination results
We assure prompt and secure access to participants’ test results:

results of imaging tests can be transferred using a professional PACS program, which connects the imaging facility with the research site
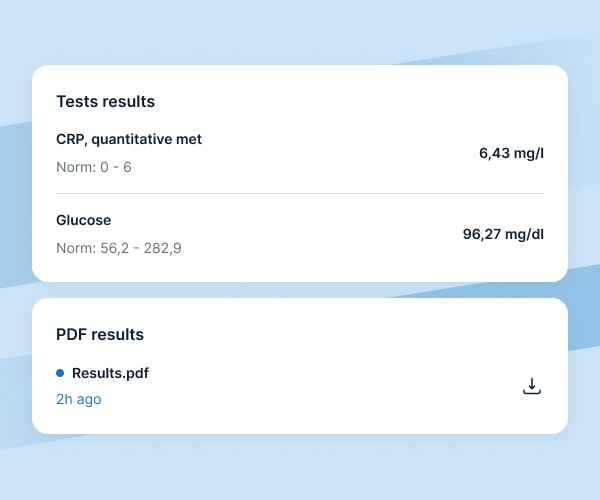
laboratory tests results are available online to research sites via HL7 integration with collections points and central laboratories
Finances
Configuring reports according to client’s preferences.
With extensive data filtering capabilities, we provide a quick access to selected parameters according to the permissions assigned by the administrator.
Vendozi ensures consolidated monthly invoicing for each billing cycle.

System security
The Vendozi platform has been developed based on the highest security standards, in collaboration with experienced researchers, clinical trial coordinators and specialists in personal data protection. We have implemented the Vendozi's good practice principles.
Compliance with the requirements of both American and European regulators regarding computerized systems, as well as the implementation of Good Automated Manufacturing Practice recommendations.
All processes executed via the Vendozi platform have been thoroughly described in SOP.
Implemented records in key areas concerning quality assurance.
Documenting activities from conception through verification, validation, software management, and integration based on the lifecycle of computerized products.
Customer support through help desk services and a library of training documents.

Quick start of cooperation
Within 24 hours, we provide comprehensive and up-to-date documentation of radiological equipment, laboratory standards and team members' CVs.
Vendozi supports the decentralization process of clinical trials by enabling the opening of centers that were previously blocked due to lack of contracts with suppliers.
We offer the possibility of remote monitoring of patients' laboratory results without the need for visits to the research sites.
Clinical trial centers gain the option to choose the most convenient locations for their patients without having to contract multiple suppliers.